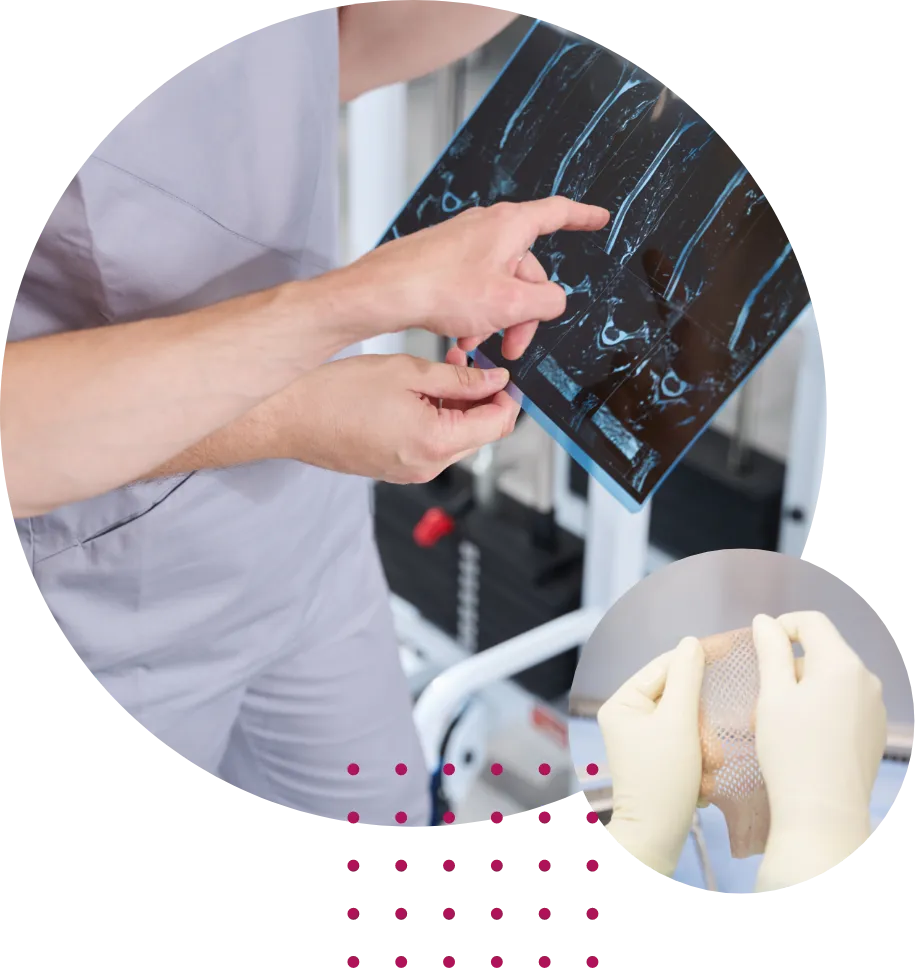
Sequence™ LifeScience, Inc. was established in 2021 with the
goal of being a premier human tissue allograft processing facility.
Working closely with partners, our skilled Operations and Quality teams provide the highest caliber human cell and tissue products (HCT/Ps). We strive to provide products with real clinical value to patients and physicians.


Long-Lasting Results

Fast, Painless Treatments

Banish Razor
Burn

Sequence LifeScience is dedicated
to ethically advancing human healing, serving our partners and community with excellence, and respecting the gift of donation. To continuously strive to achieve total customer satisfaction through the highest quality products and services. Sequence LifeScience is committed to the requirements of the Quality Management System (QMS) and to continually improving the (QMS) to maintain its effectiveness and sustainability.
Human tissue is a precious gift when donated with the unselfish hope that it may enhance another life.
Sequence LifeScience maximizes the gift of tissue donation. We treat each donors tissue with the respect and appreciation warranted to these thoughtful donors. Our human placental tissue allografts (Rebound™ Full Thickness Allograft Matrix and Emerge™ Dual Membrane Allograft Matrix) are processed in such a way to retain the structural and functional characteristics of the starting tissue. The final product is dehydrated, packaged in different size sheets, and terminally sterilized by irradiation. The product consists primarily of extracellular matrix proteins and serves as a natural, biologic barrier or wound cover.


Systematic Research- Protein Preservation & Characteristics
The structural and biochemical properties of placental tissue including extracellular matrix proteins, growth factors and anti-inflammatory cytokines make this tissue an ideal biologic for a multitude of acute or chronic wound applications (1,2).

PLACENTAL MEMBRANES:
Our current products are derived from placental tissue which was previously considered biologic waste. The placental membranes are a newly formed (during the 9 months of gestation), pristine extracellular matrix containing growth factors and cytokines produced to protect and support fetal development. This tissue essentially represents a neomatrix.Research and clinical studies going back over a century have exhibited the beneficial effects of utilizing human placental membranes to manage various types of wounds due to injury, disease, or surgical intervention. These studies have indicated several beneficial properties of the placental membranes including; serving as a protective wound barrier, anti-microbial effects and anti-inflammatory effects, among others (1,2).

DESCRIPTION OF LAYERS:
Broadly, the placental membranes include an internal or fetus facing amnion and an external or maternal facing chorion. The physical and biochemical properties of the two primary membranes are subtly different owing to the position they occupy in the placenta. The amnion is a thinner yet more compact layer of extracellular matrix while the chorion is a slightly thicker but less compact matrix. Both layers are constituted of collagen fibers (predominantly Types I, III, IV and V), along with other ECM proteins (including laminin, fibronectin, proteoglycans and hyaluronic acid) plus numerous growth factors and cytokines (2).
PROPERTIES OF PLACENTAL TISSUES
The structural and biochemical properties of placental tissue including extracellular matrix proteins, growth factors and anti-inflammatory cytokines make this tissue an ideal biologic for a multitude of acute or chronic wound applications (1,2).

All-natural biologic
The placental membrane is a newly formed extracellular matrix produced during pregnancy. In this role the membranes play a critical role in cushioning and forming a protective barrier around the developing fetus. Further, the placental membrane acts as a filter for water, soluble materials and bioactive molecules present in the amniotic fluid (3).

Anti-inflammatory
Placental membrane products have been shown to be immunogenically privileged due to a significant decrease in Major Histocompatibility Complex (4) and Human Leukocyte Antigens (HLA) (5,6).

CELL SIGNALING AND BIOLOGIC FACTORS
The placental membrane extracellular matrix also functions as a binding site for a number of cell signaling cytokines and growth factors including bFGF, EGF, HA, TGF-beta, IL-1, 6 & 10, PDGF, MMPs and TIMPs (7,8,9,10).
Our Products
Rebound™ Matrix is used by a licensed physician for single patient use. The typical patient population includes those with chronic full thickness ulcers and other skin defects where a biological barrier or wound cover is required.
Use of Emerge ™ Matrix by qualified health care professional is for application in physician office, outpatient, or inpatient setting. For a patient population where full thickness acute and chronic wounds where a biologic barrier or wound cover is required.

Rebound™ Matrix is used by or on order for a licensed physician for single patient use. The typical patient population includes those with chronic full thickness ulcers and other skin defects where a biological barrier or cover is required.



Use of Emerge™ Matrix by a qualified health care professional is for application in physician office, outpatient, or inpatient setting. For a patient population with full thickness acute and chronic wounds where a biologic barrier or wound cover is required.



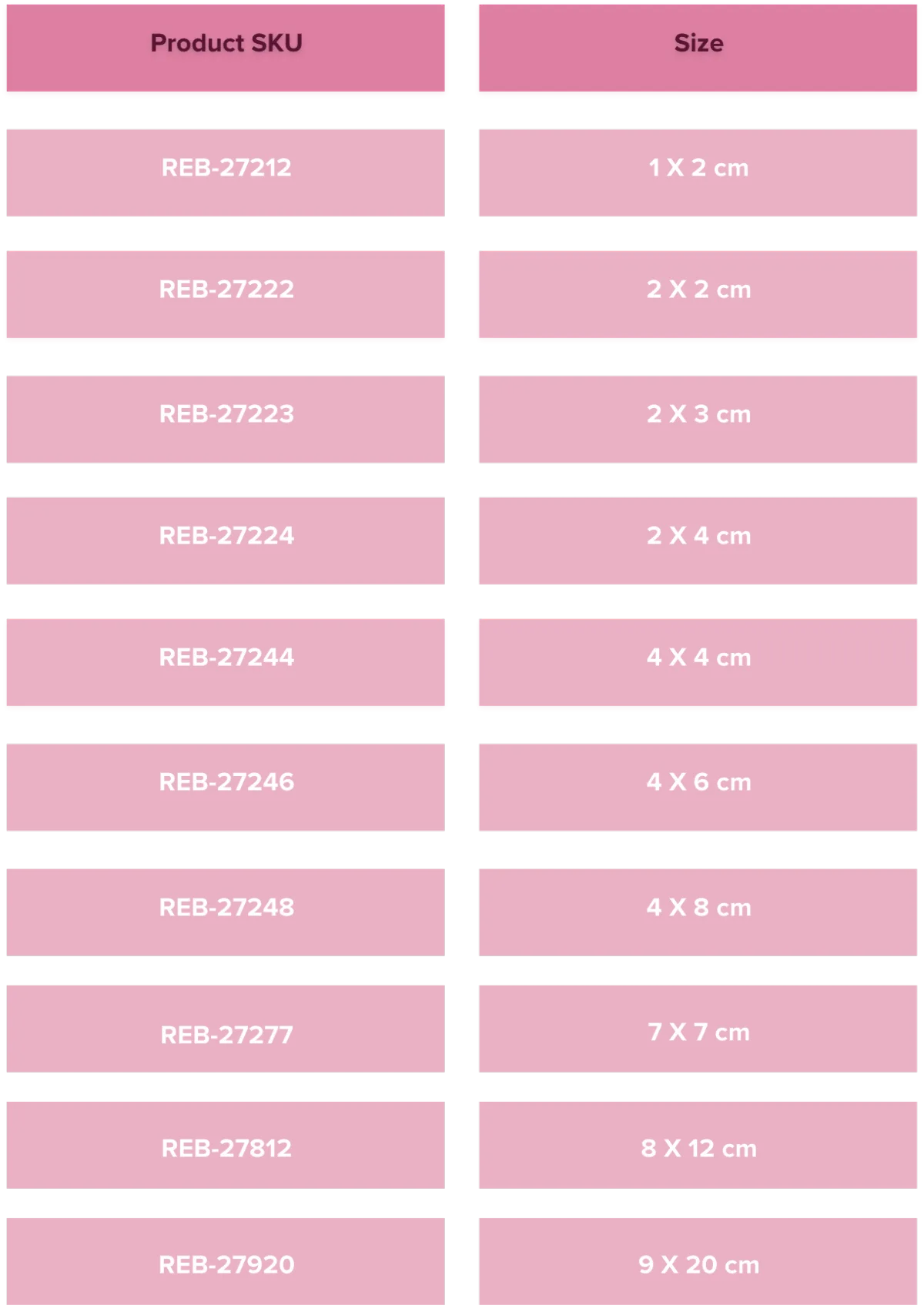
Full Thickness Placental-Derived Allograft Matrix

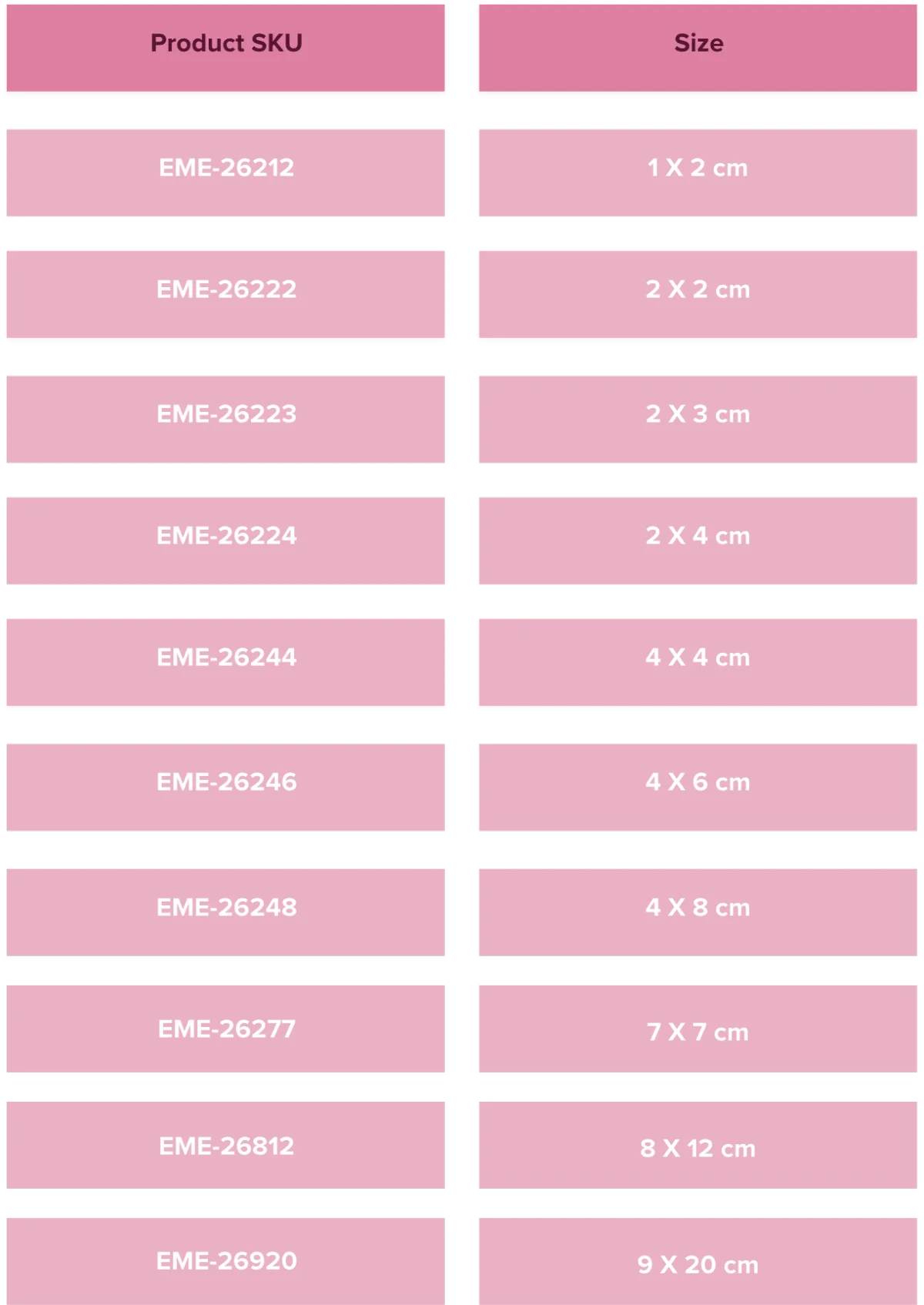
Dual Membrane Placental-Derived Allograft Matrix
Tissue processing is key
The structural and biochemical properties
of placental tissue
including extracellular matrix proteins, growth factors and anti-inflammatory cytokines make this an ideal tissue source for a biologic matrix used for a multitude of acute or chronic wound applications (11,12). Emerge™ and Rebound™ Placental-Derived Allograft tissues are processed using the Sequence LifeScience AlloKleen™ Process. The AlloKleen™ Process was developed to allow processing which maintains the structural integrity of the tissue while removing unwanted immunogenic components in the tissue. We do not use detergents or harsh chemicals during processing and have instituted extensive pre- and post-processing wash steps to remove unwanted extraneous tissues and fluids. This process allows the retention of key ECM components. The final step in the AlloKleen™ Process is a validated terminal sterilization process that ensures a 10-6 sterility assurance level (SAL) to further ensure the safety of these allograft products.
Quality & Regulatory
Dedicated to Advancing Human Healing
Our quality program takes the safety of the product very seriously and takes a quality by design approach to meet all regulatory requirements. Sequence is FDA registered for Amnion products. We partner with recovery organizations for donor screening, informed consent, collection/ acquisition of the donated tissue. Sequence ensures its partners maintain compliance with FDA and AATB. We work with our partners to prevent contamination using appropriate aseptic techniques during collection/acquisition and processing.
Sequence manufactures humans cells, tissues and cellular and tissues based products that are regulated solely under the authority of section 361 as described in 21 CFR 1271.10.
Emerge™ & Rebound™ Allograft Matrix products are processed in classified ISO 5 environments. Quality controls are in place to ensure the safety of the tissue for transplant.
Donated tissues for Sequences Allograft Matrix products are collected from fully consented mothers undergoing full term c-sections. Each donor is screened according to the strict standards required by the U.S. Food and Drug Administration and the American Association of Tissue Banks. Maternal screening includes:
Human Immunodeficiency Virus (HIV)
HIV-1/2 Antibodies (HIV-1/2-Ab)
Nucleic Acid Test for HIV-1 RNA (HIV-1 NAT)
Hepatitis B Virus (HBV)
HBV Surface Antigen (HBsAg)
HBV Core Antibody IgG & IgM (HBcAb)
Nucleic Acid Test for HBV DNA (HBV NAT)
Hepatitis C Virus (HCV)
HCV Antibody (HCVAb)
Nucleic Acid Test for HCV RNA (HCV NAT)
Syphilis*
Rapid Plasma Reagin Screen (RPR) -Or- Serologic Test for Syphilis (STS) -Or- Fluorescent Treponemal Antibody (FTA) -Or- T. pallidum IgG
West Nile Virus (WNV NAT)
Emerge & Rebound Placental-derived Allograft products undergo a validated terminal sterilization step achieving a 10-6 SAL to help ensure these products are safe for transplant.
Read OUR GLOWING REVIEWS!
This place is amazing! This is by far the best experience I’ve had with microneedling. Totally painless and the serums they use before the procedure lessen the redness of my skin afterwards. The bedside manner is unmatched and they always ask if I’m comfortable during the procedure. I’ve had 3 sessions so far and will continue coming back.
Amy Viejo

I really like the place, the service was great. The technicians explained how it works through the whole process and made me feel comfortable with the services. I purchased the laser lipo package and it's showing results after the second treatment. I am definitely coming back for the micro-needling.
Aldo Ivan Trejo Rojas

contract manufacturing
Sequence LifeScience provides contract manufacturing and working with OEMs to facilitate their production needs. Sequence LifeScience possesses cleanroom capabilities for full tissue operations such as manufacturing, sterilization, and distribution.
We also provide private label opportunities for distribution partners that require an efficient and reliable tissue manufacturer that meet or exceed expectations with quality as a crucial component

© 2024. Sequence Life Science.
All Rights Reserved.
